The landmark Tryton IDE study was a multi-national randomized trial that compared a Tryton stent in the side branch vs. the use of balloon angioplasty in the side branch, with both arms of the trial utilizing a standard drug eluting stent in the main vessel. The study, which was the first and only randomized IDE clinical trial of a bifurcation stent, enrolled over 700 patients from up to 75 centers in North America, Europe and Israel. Martin Leon, M.D. (Columbia University, New York) served as principal investigator for the study and Patrick Serruys, M.D., PhD. (Thoraxcenter, Rotterdam) led the IVUS.
Outcomes patient pooled analysis - Konigstein_et al. CCI 2018
Tryton 5 year clinical outcomes - P. Green et al - CRM 2018 in press
Tryton dedicated bifurcation stent in treatment of unprotected distal left main bifurcation disease
Outcomes of Tryton in Bifurcations with Large Side Branches – RCT Post Hoc Analysis
Dedicated Bifurcation Stent for the Treatment of Bifurcation Lesions Involving Large Side Branches
Outcomes of Tryton in Bifurcations with Large Side Branches – RCT Post Hoc Analysis
Tryton Randomized Trial
6M And 1Y Clinical Outcomes: A Patient-Level Pooled Analysis Of 8 Registry Studies
2-Year Clinical Follow-up of the Tryton IDE Randomized Trial
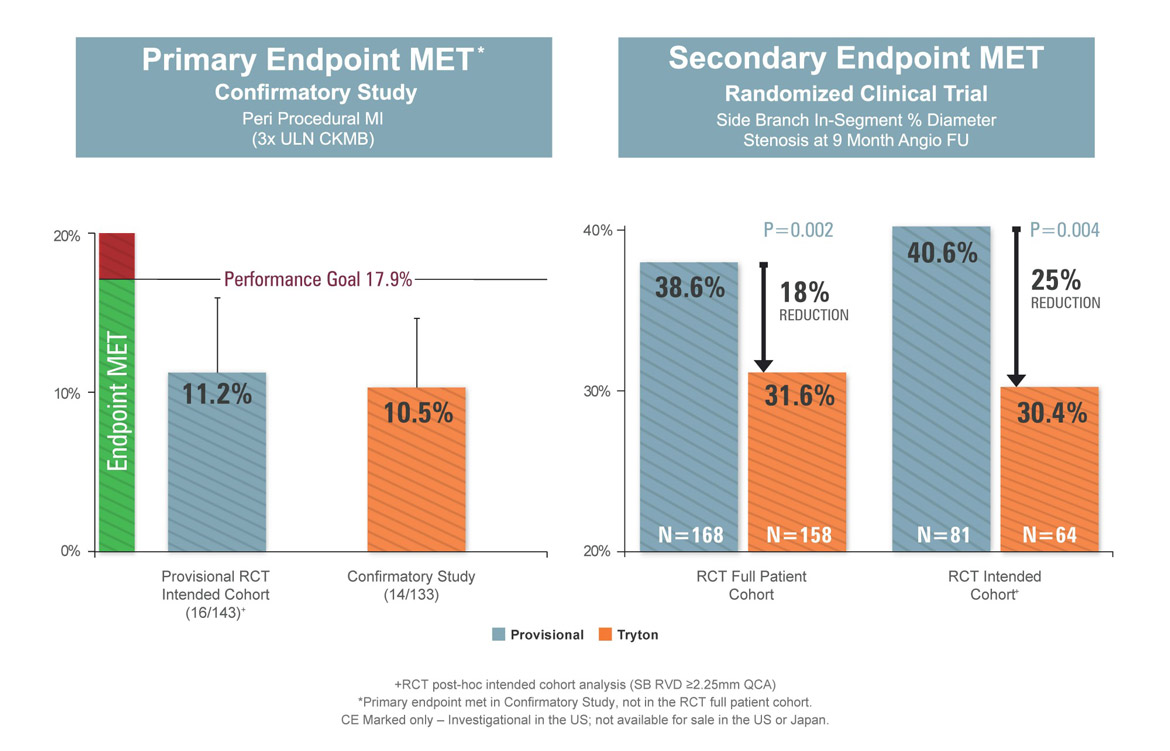
Incidence of Peri-Procedural MI: Insight from the Tryton IDE Randomized Trial
Stent Expansion and Lesion Coverage Insights From Intravascular Imaging | Antonio L. Bartorelli, M.D.
Lesion Coverage: Insights from the Bench Top | Maciej Lesiak, M.D.
Tryton – Addressing the Challenge of Bifurcation Stenting | Dean J. Kereiakes, M.D.
Dedicated Bifurcation Stent Technology: Implications for Everyday Practice | Jens Flensted Lassen, M.D.
Bifurcation Lesion Treatment Option: Insights From Clinical Literature | James Hermiller, M.D.
Tryton Pivotal: Randomized Trial and Confirmatory Study - Key Messages | Martin B. Leon, M.D.
The Tryton Pivotal: Randomized Trial & Confirmatory Study | Philippe Généreux, M.D.






